nitrogen protons neutrons electrons|2.6: Protons, Neutrons, and Electrons in Atoms : Baguio 1K. 88K views 5 years ago The Atom: Protons, Neutrons, Electrons, and Mass Number. In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and.
Welcome to Fly Away Simulation - your trusted and well-known flight simulator portal that's been in the industry for over 20 years.Launched in 1999 as a flight sim enthusiasts portal providing downloads and add-ons. Now trusted by over 200,000+ PRO subscribers and over 500,000 new visitors visiting our website each month. .In Medieval 2 the max number of units per stack is actually 20, not 16, and the max unit size is 150 on 'huge' setting. That makes 3000 maximum per side, so 1 vs 1 would be 6000 men. In Empire we can expect to see the unit sizes stay the same or increase, so I would say 150 is most likely, and anywhere up to 200 is at least possible.
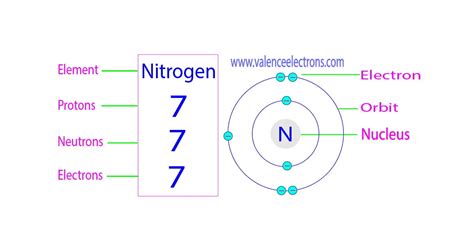
nitrogen protons neutrons electrons,Nitrogen-16 is composed of 7 protons, 9 neutrons, and 7 electrons. In nuclear reactors, nitrogen-16 can be used to detect leakages from steam generators. Nitrogen-16 is an isotope of nitrogen .Learn about the three types of subatomic particles that make up atoms: protons, neutrons, and electrons. See how they are charged, massive, and located in the nucleus or the electron cloud of an atom.

Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons in an atom. Write and .
Describe the locations, charges, and masses of the three main subatomic particles. Determine the number of protons and electrons in an atom. Write and .The mass of an atom relative to that of carbon-12. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, .Nitrogen is the 7th element in the periodic table and has a symbol of N and atomic number of 7. It has an atomic weight of 14.007 and a mass number of 14. Nitrogen has seven . 1K. 88K views 5 years ago The Atom: Protons, Neutrons, Electrons, and Mass Number. In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and.
Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in . Neutral atoms have the same number of electrons and protons. Atoms of an element that contain different numbers of neutrons are called isotopes. Each isotope of a given element has the same .An element is defined by the number of protons in its atoms, known as its atomic number. The periodic table organizes the elements by atomic number and provides other key information about them such as their names and chemical symbols. Created by Khan .Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an atom to have 8 total . The charge of an atom is defined as follows: Atomic charge = number of protons − number of electrons (1.8.1) (1.8.1) Atomic charge = number of protons − number of electrons. As will be .
An early model of the atom was developed in 1913 by the Danish scientist Niels Bohr (1885–1962). The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun.GCSE; CCEA; Atomic structure - (CCEA) Protons, neutrons and electrons Scientists’ ideas about atoms have changed over time. Today, they agree that atoms have a positively-charged nucleus made of .
Nitrogen is found to have either 3 or 5 valence electrons and lies at the top of Group 15 on the periodic table. It can have either 3 or 5 valence electrons because it can bond in the outer 2p and 2s orbitals. . Oxides of nitrogen are acidic and easily attach protons. \[N_2O_5 + H_2O \rightarrow 2HNO_{3 (aq)} \label{5} \] The oxides play a .
Neutral carbon-14 contains six protons, eight neutrons, and six electrons; its mass number is 14 (six protons plus eight neutrons). These two alternate forms of carbon are isotopes. . so since the carbon 14 with 6 protons and 8 neutrons when one of the neutrons becomes a proton it has 7 protons and 7 neutrons, the 7 protons makes it . Number of Neutrons = Mass Number - Number of Protons = 1 - 1 = 0. For zinc, the atomic weight is 65.39, so the mass number is closest to 65. Number of Neutrons = 65 - 30 = 35. Cite this Article. Follow these simple steps to find the number of protons, neutrons, and electrons for an atom of any element.
L’atome est donc essentiellement composé de vide. Ce qu’il faut retenir : la masse d’un proton et d’un neutron est quasiment la même. Mais un électron est environ 1000 fois plus léger : la masse des électrons est donc négligeable par rapport à celle des protons. Ainsi la masse de l’atome est concentrée dans le noyau. Table 4.4.1 4.4. 1 gives the properties and locations of electrons, protons, and neutrons. The third column shows the masses of the three subatomic particles in "atomic mass units." An atomic mass unit (amu amu) is defined as one-twelfth of the mass of a carbon-12 atom. Atomic mass units ( amu amu) are useful, because, as you can .

Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The chemical symbol for Nitrogen is N. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons.Going up the elements, protons increase by 1 and electrons also increase by 1. - Shell 1 can only hold a maximum of 2 electrons. - Shell 2 can hold up to 8 electrons. - The third shell can hold 8 .2.6: Protons, Neutrons, and Electrons in AtomsWe recommend using the latest version of Chrome, Firefox, Safari, or Edge. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test . Mass Number = # of Protons + # of Neutrons. Mass Number = 1 + 2. Therefore, this particular atom of hydrogen will have a mass number of 3. Note that the mass number calculated in Example .nitrogen protons neutrons electrons 2.6: Protons, Neutrons, and Electrons in Atoms Atomic number or Protons: 7: Neutrons: 7: Electrons: 7: Symbol: N: Atomic mass: 14.007 u: . Nitrogen has 5 electrons in its outermost orbit and it needs 3 more electrons to complete the octet configuration. Thus the nitrogen atom combines with the other nitrogen atom by sharing 3 electrons. In this way, it forms three covalent .
Nitrogen is an atom that has 7 protons, 7 neutrons, and 7 electrons. The neutrons and protons are all stuck together in the middle of the atom and are called the nucleus. The 7 electrons are much much smaller than the nucleus and spin around it in things called orbits. The inner orbit contains two electrons, and the other 5 electrons are in the .nitrogen protons neutrons electrons Mass numbers of typical isotopes of Nitrogen are 14; 15. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A. The difference between the neutron number and the atomic number is known .
Make sure that you round the atomic mass to the nearest whole number. For example, the atomic mass of boron is 10.811, but you can just round the atomic mass up to 11. 6. Subtract the atomic number from the atomic mass. To find the number of neutrons, you will need to subtract the atomic number from the atomic mass. The number of neutrons can be found by subtracting the atomic number from its atomic mass. Number of Neutrons in Nitrogen = Atomic mass of Nitrogen – Atomic number of Nitrogen = 14 – 7 = 7. Number of Electrons in Nitrogen. For a neutral atom, the number of electrons can be found by knowing the atomic number of that atom.
nitrogen protons neutrons electrons|2.6: Protons, Neutrons, and Electrons in Atoms
PH0 · Protons, neutrons, and electrons in atoms (video)
PH1 · Nitrogen (N)
PH2 · Nitrogen
PH3 · How to find the Number of Protons, Electrons, Neutrons for
PH4 · How to find the Number of Protons, Electrons, Neutrons for
PH5 · Elements and atomic number (video)
PH6 · 8.9.2: Chemistry of Nitrogen (Z=7)
PH7 · 2.6: Protons, Neutrons, and Electrons in Atoms
PH8 · 1.8: Subatomic Particles